Alzheimer’s Disease (AD) is a neurological disease that affects specific brain regions in a deterministic fashion over time, and clinically leads to a cascade of symptoms that ultimately result in a loss of cognitive ability. Traditionally, AD was definitively diagnosed only upon autopsy through the identification of hallmarks of the disease – extracellular aggregates called amyloid plaques and intracellular aggregates called tau tangles - using imaging techniques. It was discovered that a certain percentage of patients with significant AD pathology never experienced a loss of cognitive ability during life, suggesting there were mechanisms at play which protect them from the clinical effects of AD. Studying the basis of how this phenomenon – termed cognitive resilience – occurs is an exciting area of research that may lead to new therapeutic avenues, as identified mechanisms may be able to be upregulated in patients at risk of developing AD.
We are investigating these mechanisms in animal models that vary along how much resilience they show to amyloid plaques. These models are part of the AD-BXD panel of cognitive resilience developed by Dr. Catherine Kaczorowski at JAX, and are genetically distinct from each other. Assuming a controlled environment, any differences in the cognitive resilience of these mice should only be due to changes in the genetic makeup of the animals.
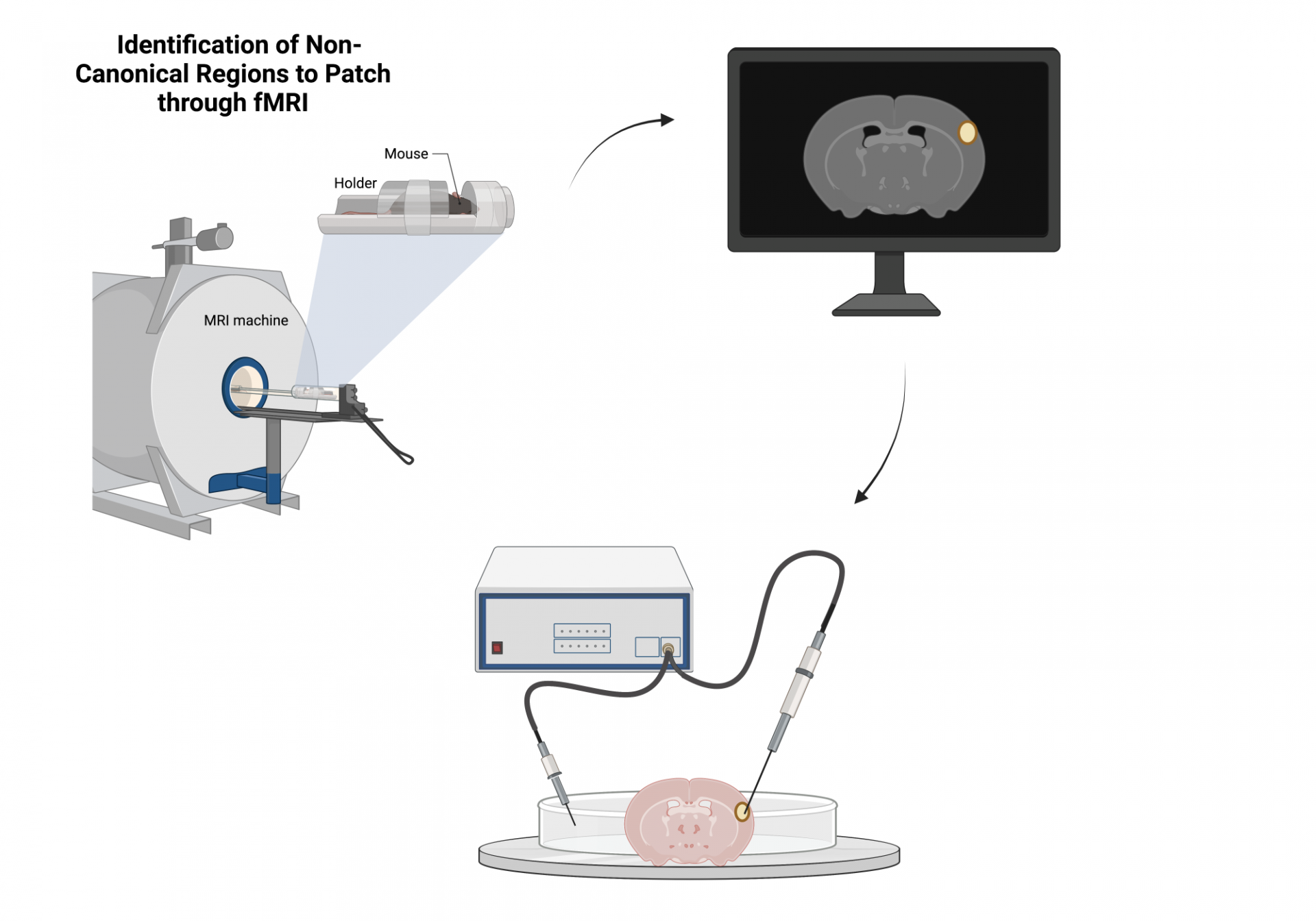
The method we use – resting state fMRI – provides information on patterns of neural activity between brain regions. The lab of one of our collaborators, Dr. Gagan Wig, found that changes to one metric derived from these patterns - called Brain System Segregation (BSS) - is predictive of future cognitive decline in humans. As a first step, we are identifying whether these results extend to our animal models of resilience. We predict that resilient strains will have lower changes to BSS than non-resilient ones. We will then perform targeted interventions in order to see whether we can affect changes to BSS by changing parameters thought to be related to the development of AD, such as levels of social activity or exercise.
Additionally, our analyses will be used to identify brain regions where neural activity differs between resilient and non-resilient animals. These results will be followed up by experiments in the labs of some of our collaborators: Drs. Catherine Kaczorowski, Kristen O’Connell, and Vilas Menon, to identify gene expression or electrophysiological changes of specific cell types between resilient and non-resilient strains.
Group Members: Eyal Bergmann and Jonathan Algoo